Alright, so I’ve been seeing this orbital stuff popping up lately, especially folks getting tripped up trying to tell 4f and d orbitals apart. Figured it was time I sat down myself and just did it, you know? Got my hands dirty. Real simple goal: see if I could spot the key differences fast.
First Step: Pulling Out the Basics
Started by grabbing my old, dusty chemistry notes. Honestly felt like archeology. No fancy textbooks, just the stuff I scribbled years ago. Knew I needed pictures, something visual to make sense of these fuzzy electron cloud things. Tried sketching a ‘d’ orbital first from memory – kinda looked like a wonky four-leaf clover.
Where I Messed Up (Big Time)
Then I went for the ‘f’ orbital. Disaster. Brain went completely blank. My sketch looked suspiciously like a misshapen potato with lobes sticking out weirdly. Worse, I started confusing the ‘f’ shapes with some of the weirder ‘d’ shapes. Totally jumbled in my head! Needed a reset.
Actually Looking Stuff Up
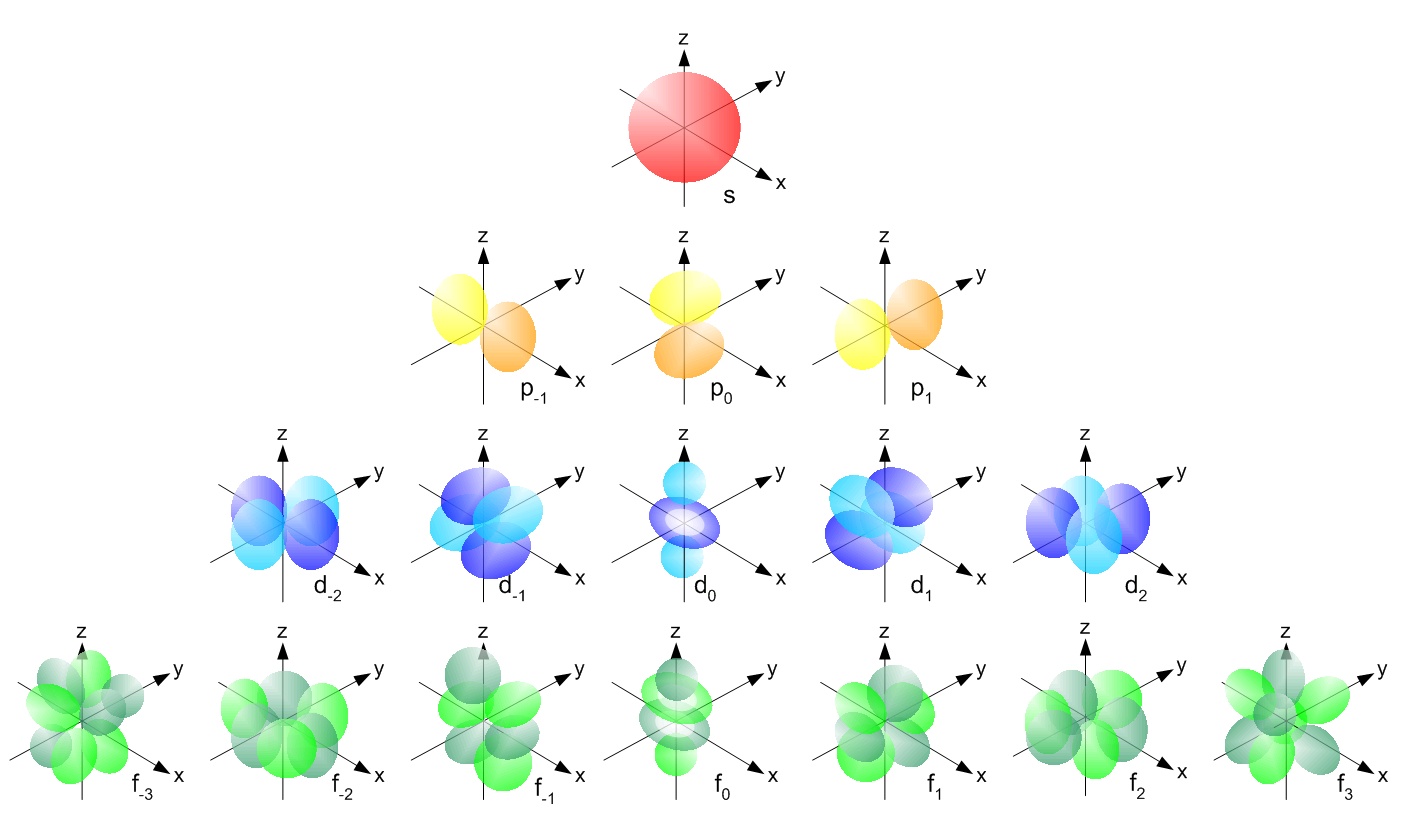
Okay, fine. Pulled up some simple diagrams online (no fancy sites, just image search). Just stared at them side-by-side: ‘d’ orbitals vs ‘4f’ orbitals. Ignored the hardcore math, just focused on what my eyes could tell me right then and there.
Here’s what actually jumped out at me:
- The Shape Count Thing: Pretty obvious once I saw it. ‘d’ orbitals? Mostly like variations on four clover leaves. ‘4f’ orbitals? Way more complicated – honestly looked like eight pointy bits popping out in all directions. That was difference number one locked down.
- Where They Hang Out: I traced my finger over where each type sits in an atom. ‘d’ orbitals? They’re like the main suburbs surrounding the nucleus – pretty close in, starting row four. ‘4f’ orbitals? Deep backwoods! Way further out, buried past the ‘d’s, like hiding behind them. Difference number two – location, location, location.
- First Timer or Not: Thinking about elements showing these orbitals for the first time. For ‘d’ orbitals, they debut much earlier in the cast, like right at Scandium (Sc) in row four. ‘4f’ orbitals? They come super late to the party! Doesn’t happen until the Lanthanides way down in row six. Important difference for knowing when they show up.
- What’s The Point? (Electrons) Then I just counted how many seats each orbital type has. Simple math: each orbital holds 2 electrons max. ‘d’ block? That’s 5 orbitals total – so room for 10 electrons max. ‘f’ block? Whew, that’s 7 orbitals! Bigger house, room for 14 electrons. That was the fourth, simple difference.
Putting It Together & My Main Takeaway
So yeah, by actually looking at the pictures and focusing on basic stuff I could see and count without going deep into quantum physics:
- Count the lobes/points (4ish vs 8ish).
- Check their address in the atom (closer suburbs vs deep backwoods).
- See when they first appear on the periodic table block (early row 4 vs way late row 6).
- Max electron capacity per block (10 vs 14).
It clicked. Didn’t need a PhD, just needed to do the simple comparison myself. It’s easy to overcomplicate this stuff. Sometimes you just gotta grab a pencil and sketch it badly, look it up, and spot the obvious bits staring you in the face. Works way better than memorizing pages of text.