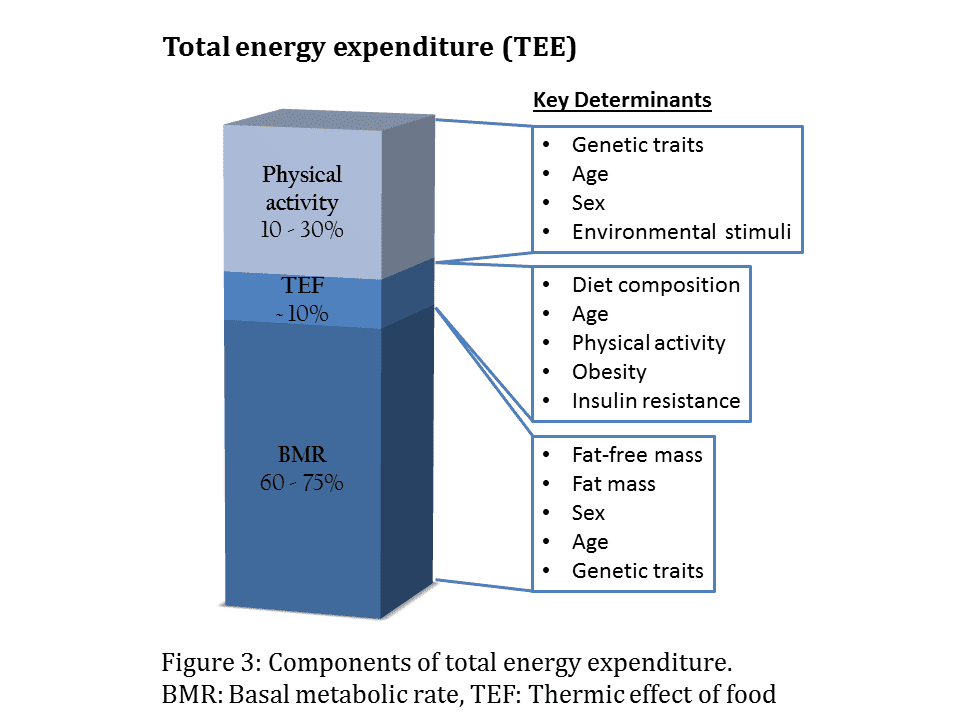
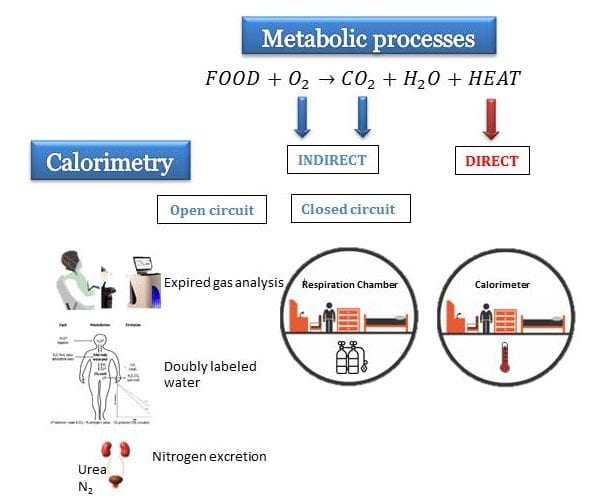
Okay, so today I wanted to figure out how heat energy is measured indirectly. It’s something I’ve been curious about, and I thought, why not dive in and see what I could find?
Getting Started
First, I did some basic searching online. I wanted to get a handle on the whole concept. Turns out, you don’t really measure heat energy directly. It’s always about measuring something else and then figuring out the heat from that. Kind of like detective work!
My Little Experiment
I decided to do a simple experiment at home. Nothing fancy, just something to help me understand the idea.
- I got a pot of water.
- I grabbed a thermometer, you know, the regular kind for checking if you are fever.
- I also used a kitchen scale to measure the water.
- And, of course, the stove to heat things up.
The Process
I carefully measured out some water and put it in the pot. Then, I took the initial temperature. Easy peasy. Next, I turned on the stove and let the water heat up for a bit. After a few minutes, I turned off the stove and took the temperature again.
The temperature had gone up, obviously. This is where the “indirect” part comes in. The thermometer didn’t measure heat energy directly. It measured the change in temperature of the water. And from that change, along with knowing how much water I had, I was able to calculate how much the heat energy content of water changed!
Wrapping Up
So, that’s basically it! Heat energy is measured indirectly by observing changes in other things, like temperature. It’s all about figuring out the heat based on those other measurements. I found it pretty cool to see it in action, even with a simple experiment like this. I think there are many ways to do so. Maybe I’ll try a different method next time!